Alzheimer's disease/ cancer/cardiovascular disease/diabetes/kidney stone/Parkinson's.... |
Mechanisms of Action of IP6 & Inositol
IP6 has been best known as an antioxidant, "anti-nutrient" label notwithstanding. In our body highly reactive toxic chemicals such as •OH (hydroxyl radicals) are formed as a consequence of oxidation of Fe2+ to Fe3+ during the reaction of Fe2+ with H2O2 via Fenton Reaction. These radicals react with DNA, proteins or lipids causing cell injury or even cell death; beginning from ageing to Alzheimer's disease, cancer, myocardial damage during infarction, etc have been incriminated to them. |
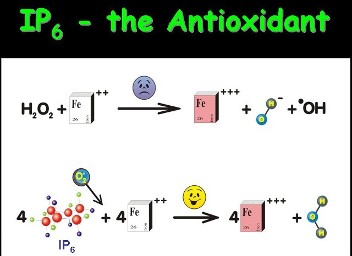 |
By binding with and hence inactivating Fe2+, IP6 prevents the formation of toxic •OH. Inas-much as oxidative damage is harmful to us, it is no less dangerous for our enemies - the microbes and cancer. Radiotherapy-induced killing of cancer cells is mediated via oxidative damage and so is the destruction of bacteria by our 'foot soldier' cells the polymorphonuclear neutrophils. As in normalization of cell proliferation (see below), in here too, IP6 while one one hand acts as an anti-oxidant to protect us from the harmful free radicals, it also enhances our body's ability to kill the invading microbes through increased production of the superoxides inside the neutrophils! |
 |
Another of the mechanisms of action of IP6 and inositol is inhibition of excessive and uncontrolled cell growth (normalization) in disease conditions without affecting the rate of cell growth in normal cells. In very high doses, IP6 may kill cancer cells, but not normal cells, a distinct difference with most anti-cancer agents. |
During transformation of normal cells to malignancy, the cancer cells lose the normal behavioral pattern, a phenomenon called de-differentiation. Concomitantly, they also look different: the cancer cells have larger cell body (cytoplasm) and disproportionately large nucleus, i.e. increased nuclear : cytoplasmic ratio. Another broad mechanism of action of IP6 & Inositol is by reversing that process or causing cell differentiation back to normal, so they also begin to look like normal cells. The panel below illustrates both the inhibition of cell growth and induction of differentiation in a human mammary cancer cell line. |
 |
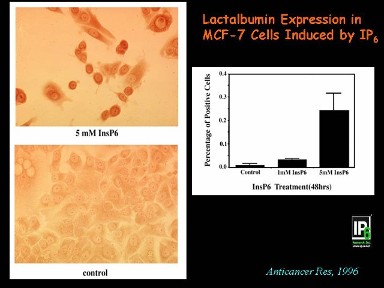 |
The photomicrograph at the bottom left is crowded with MCF-7 human breast cancer cells (Control). Treatment with IP6 results in decreased cell number as well as increased differentiation as dark brown coloration (upper left panel) representing increased lactalbumin production - a marker of normal mammary epithelial cells. The bar-graph shows dose-response increase in lactalbumin production. Note that there is no evidence of cell death (Anticancer Research 1996). |
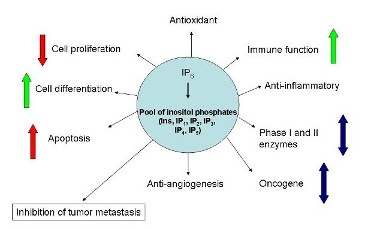 |
Additionally, there are many other mecha-nisms by which IP6 & Inositol exert their action. IP6 may be dephosphorylated to inositol and IP1-5 or acquire additional phosphates (pyrophosphates) to become IP7&8 who in turn could be the mediators of the actions listed to the left. Several groups of investigators have shown that IP6 also acts as an anti-angiogenesis agent, one of the molecular mechanisms may be by binding with FGF1. |
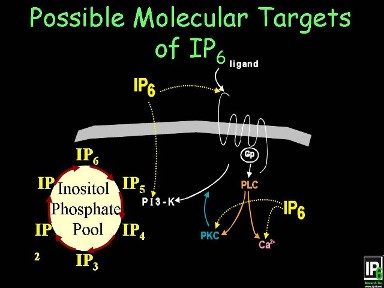 |
Cell surface receptors for IP6 have been identified on various different cell types. Some of the functions of IP6 could very well be receptor-mediated, effecting the down-stream signaling molecules and releasing intracellular Ca++, the latter triggers many intracellular behaviors such as cell division, cell differentiation, etc. It may also enter into the intracellular inositol phosphates pool with yet to be determined fate and function. |
Epigenetic changes, the early steps in carcinogenesis involving gene expression without affecting DNA sequence modification are no less important. DNA methyl transferases, methyl CpG binding proteins, methyl CpG DNA binding domain protein, and histone deacetylases are the major molecules involved in epigenetics. In the December 2010 issue of the Journal Nutrition & Cancer Drs Pandey and Gupta report that in their mouse-lung tumorigenesis model, while the carcinogen ENU up-regulated the epigenetic events such as the expressions of DNMT1, MeCP2, MBD1, and HDAC1, these alterations were reduced by IP6 administration. |
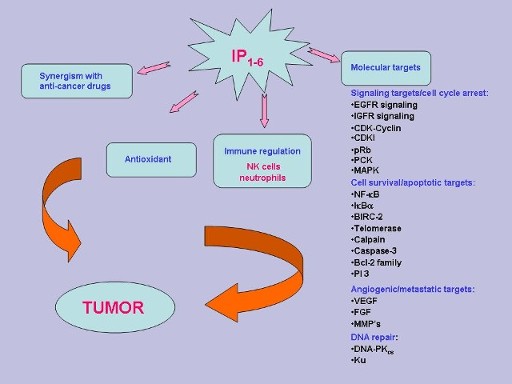 |
Ongoing research are providing us with new data about how IP6 modulates other intracellular regulators such as p53, p27, PI-3 Kinase, NFκB, PKCδ, ppRb, Akt, ERK 1/2 etc. These are some of the pathways by which IP6/Inositol acts. For the latest research papers please go to PubMed and key in "ip6" or "InsP6" or "inositol hexaphosph-ate" or "inositol hexa-kisphosphate" |
|
|